Best Pharmaceutical Disposal Companies: Safe & DEA-Compliant
What Is a Pharmaceutical Disposal Company? A pharmaceutical disposal company helps healthcare providers, retail pharmacies, hospitals, and long-term...
4 min read
Lori Tanner : May 25, 2025 10:49:06 AM
Across the United States, healthcare providers, pharmacies, labs, and long-term care centers must safely manage the destruction of controlled substances. These include expired narcotics, damaged opioids, and unwanted controlled substances that can no longer be used.
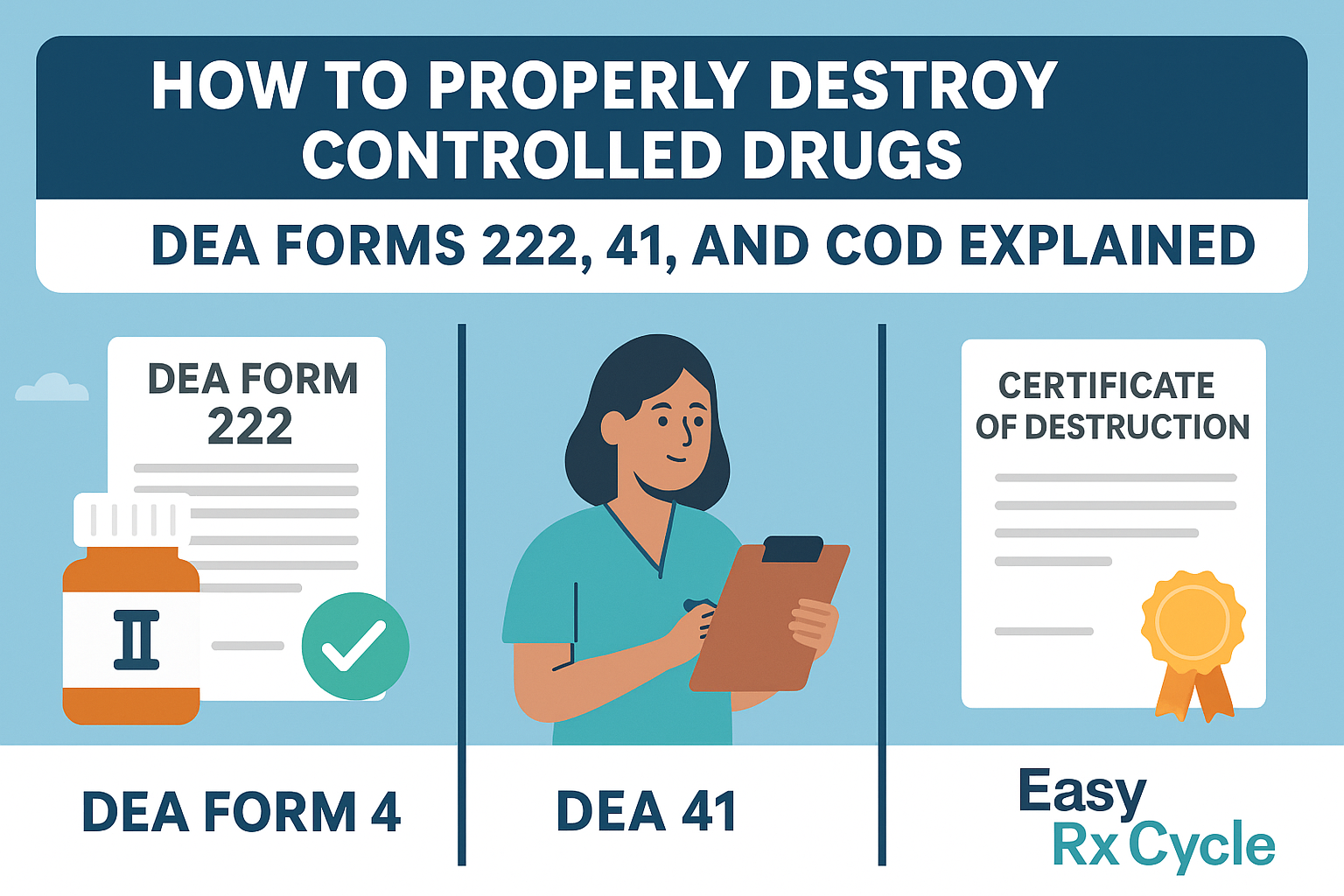
Because these substances are highly regulated, the Drug Enforcement Administration (DEA) requires facilities to follow strict procedures for disposal—using DEA Form 222, DEA Form 41, and a Certificate of Destruction (COD) to remain compliant with federal regulations under 21 CFR.
Failure to properly dispose of controlled substances can result in DEA audits, fines, loss of licensure, and even criminal liability. These forms protect the public, ensure safety, and maintain the legal chain of custody.
Looking for the Best Pharmaceutical Disposal Companies: Safe & DEA-Compliant?
What are the Best Drug Disposal Companies: Safe & DEA-Compliant or the Best Reverse Distributors?
DEA Form 222 is required for the transfer of Schedule I and II controlled substances, including those marked for destruction. Whether transferring for use or disposal, this DEA form serves as an official record of controlled substances being moved from one registrant to another.
It is also part of the registrant record of controlled substances destroyed, providing clear documentation under DEA and 21 CFR rules. Need help? Request a Quote!
DEA Form 41—also known as the controlled drugs destruction form—is used to document the method of destruction for expired or unwanted controlled substances. This form is required for DEA registrants who are destroying drugs directly or through an authorized third party.
Please note: If your facility uses a reverse distributor, the distributor may file the Form 41 on your behalf. However, you’re still responsible for recordkeeping. Got Questions? Request Help Now!
A Certificate of Destruction (COD) is issued by your pharmaceutical waste disposal vendor or reverse distributor after the drugs are physically destroyed. It is proof that the substance disposal process was safe and secure, conducted in compliance with DEA, EPA, and state laws.
Together, DEA Forms 222 and 41, plus the COD, form a full compliance trail for all DEA registrants involved in the destruction of controlled substances.
Follow this process to ensure you meet all federal regulations and stay compliant with the Drug Enforcement Administration (DEA):
These forms are required for many types of DEA registrants, including:
Any DEA registrant in the United States that handles controlled substances must document how they dispose of controlled substances using approved medication disposal forms and follow the substance disposal protocols in 21 CFR.
In addition to DEA rules, facilities must also comply with Environmental Protection Agency (EPA) guidelines under the Resource Conservation and Recovery Act (RCRA) for any pharmaceutical waste that may be classified as hazardous.
This dual oversight ensures that all drug waste—whether hazardous, non-hazardous, or controlled—is disposed of in a safe and secure manner that protects human health and the environment.
At Easy Rx Cycle, we help you stay compliant with all controlled substance disposal regulations. We manage every step, from providing containers to handling forms, pickups, and destruction documentation.
We make the destruction of controlled substances simple, traceable, and legally sound.
Don’t risk non-compliance. Partner with Easy Rx Cycle for safe and secure controlled substance disposal. We’ll handle the paperwork, pickups, and destruction—and make sure your facility passes any DEA or state inspection.
Contact us now for a free consultation or to schedule your next destruction pickup.
What is DEA Form 41 used for?
DEA Form 41 is used to record the destruction of controlled substances, including method, quantity, and witnesses. It is required for on-site disposal or through authorized vendors.
Do I need both DEA Form 222 and Form 41?
Yes, if you are transferring Schedule I–II substances, you’ll use Form 222. You’ll also need Form 41 to document destruction.
What is a form 41 pharmacy?
A “form 41 pharmacy” refers to any registered facility that regularly uses DEA Form 41 to destroy expired or unwanted drugs.
How do I get a Certificate of Destruction?
Your licensed destruction partner will provide a COD after the drugs are safely destroyed.
Are there federal regulations for all drug disposal?
Yes. DEA, EPA, and state boards regulate substance disposal to ensure that all DEA registrants dispose of controlled substances in a compliant and environmentally safe manner.
Request a Quote Today!
What Is a Pharmaceutical Disposal Company? A pharmaceutical disposal company helps healthcare providers, retail pharmacies, hospitals, and long-term...

What Is a Drug Disposal Company? A drug disposal company provides safe, compliant solutions for medication disposal and the handling of expired,...

Master Guide to Controlled Substance Disposal: Federal Compliance, Environmental Safety, and Risk Mitigation