How to Dispose of Expired Controlled Substances: A Complete Guide for Healthcare Facilities
Safe Disposal of Expired Drugs for Healthcare Facilities Proper drug disposal isn't just a best practice—it’s the law. If you work in a hospital,...
27 min read
William Doxey
:
May 24, 2025 3:29:05 PM

Every healthcare organization—from hospitals and pharmacies to veterinary clinics and biotech labs—generates pharmaceutical waste. This includes expired medications, contaminated supplies, and hazardous waste pharmaceuticals that require strict handling.
Improper disposal isn’t just dangerous—it’s illegal. The Environmental Protection Agency (EPA), Drug Enforcement Administration (DEA), and state agencies enforce complex waste regulations under the Resource Conservation and Recovery Act (RCRA). If you're not managing and disposing of your waste properly, you risk costly fines and serious environmental consequences.
At Easy Rx Cycle, we help healthcare providers across the Southeast—Florida, Georgia, Alabama, Arkansas, South Carolina, and beyond—build simple, cost-effective, and compliant systems for pharmaceutical waste disposal.
This guide will walk you through everything you need to know about:
Types of pharmaceutical waste
Who generates it
Disposal methods
DEA and EPA rules
2025 compliance updates
Proven waste management practices
Let’s break it all down.

Pharmaceutical waste includes any medication, drug compound, or product used in healthcare that is no longer usable or needed. This covers everything from disposal of unused drugs, to expired, contaminated, or partially used products.
Pharmaceutical waste falls under a broader category of medical waste, but its disposal practices must meet far more specific waste regulations.
Facilities that generate pharmaceutical waste must comply with the Resource Conservation and Recovery Act (RCRA) and state-level laws that govern how to properly dispose of hazardous pharmaceutical waste, controlled substances, and non-hazardous meds.
Understanding the types of pharmaceutical waste is essential for proper sorting and compliance. Here's a breakdown of common categories:
Hazardous pharmaceutical waste includes any drug or medication that exhibits characteristics of toxicity, ignitability, corrosivity, or reactivity, as defined by the Resource Conservation and Recovery Act (RCRA) and regulated by the Environmental Protection Agency (EPA). These substances pose significant risks to public health and the environment if not managed properly.
Hazardous pharmaceutical waste falls into two main classifications:
P-listed wastes: Acutely hazardous substances found in unused form (e.g., warfarin, nicotine).
U-listed wastes: Toxic substances typically discarded as residues in containers or as cleanup materials.
Warfarin (Coumadin) – A widely used anticoagulant, listed under both P and U codes depending on concentration.
Epinephrine – A drug used to treat severe allergic reactions, toxic at certain thresholds and classified as P042.
Nicotine – Present in smoking cessation products; highly toxic and acutely hazardous.
Chemotherapy Drugs – Many antineoplastic agents (e.g., cyclophosphamide, daunorubicin) are RCRA hazardous due to their carcinogenic or mutagenic properties.
Toxicity: Poses a risk to human health or the environment even in small amounts.
Ignitability: Can easily catch fire (e.g., alcohol-based solutions).
Corrosivity: Capable of corroding metals or destroying tissue (e.g., strong acids or bases).
Reactivity: Can explode or release toxic gases when mixed with water or under heat.
Proper waste management of hazardous pharmaceuticals is not optional—it is a legal obligation for all healthcare generators. Here’s what’s required to stay compliant:
Proper Labeling
All hazardous pharmaceutical containers must be clearly labeled with:
Hazard class symbols (e.g., flammable, toxic)
Contents and date of accumulation
“Hazardous Pharmaceutical Waste” designation
Segregation and Storage
Must be stored in dedicated, leak-proof containers that are:
Chemically compatible with the waste
Located in a secure, restricted area
Not mixed with non-hazardous or biohazardous waste
Licensed Transportation
Hazardous pharmaceuticals must be shipped using licensed hazardous waste transporters that:
Provide full manifests
Follow DOT and EPA shipping protocols
Ensure cradle-to-grave tracking of waste
Certified Disposal
Most hazardous waste pharmaceuticals must be incinerated at high temperatures in RCRA-permitted facilities. Landfilling or sewer disposal is strictly prohibited.
Documentation and Compliance Logs
Facilities must maintain:
Waste manifests
Inventory records
Transport receipts
Disposal Certificates (CODs)
Emergency plans in case of a spill or exposure
Hospitals (especially ERs, oncology, and ICU units)
Surgery centers (use of ignitable anesthetics)
Oncology clinics (antineoplastics)
Retail and mail-order pharmacies (expired drugs)
Veterinary hospitals (toxic flea/tick meds, euthanasia agents)
Failing to manage hazardous drug waste properly can result in:
EPA fines exceeding $70,000 per day per violation
Civil and criminal penalties for willful neglect
Contamination of water supplies and community health risks
Non-hazardous pharmaceutical waste refers to medications and related products that are not classified as hazardous under the EPA's Resource Conservation and Recovery Act (RCRA), but still pose environmental, public health, and legal risks if improperly disposed of. These substances are not ignitable, corrosive, toxic, or reactive, but they often contain active ingredients that can disrupt ecosystems or be misused if diverted.
Though less regulated than their hazardous counterparts, these drugs still require responsible handling under state laws, local ordinances, and best practices in pharmaceutical waste management.
Saline and IV fluids – Sodium chloride and lactated Ringer’s solutions are non-hazardous but should never be flushed.
Antibiotics – Medications like amoxicillin or doxycycline are not EPA-listed but can contribute to antibiotic resistance if released into the environment.
Over-the-counter medications – Includes pain relievers (e.g., ibuprofen, acetaminophen), cold medicines, and allergy relief products.
Topical creams and ointments – May not meet hazardous waste definitions but still require safe disposal to prevent contact contamination.
Expired vitamins and supplements – Not classified as hazardous but must still be managed to prevent accidental ingestion or environmental contamination.
Even though the EPA doesn’t classify these substances as hazardous, improper disposal—such as flushing down the sink or tossing in regular trash—can lead to:
Drugs entering water systems, impacting wildlife and drinking water supplies
Wastewater treatment plants unable to filter pharmaceutical compounds
Community health risks from improper access or diversion
Violations of state-level disposal laws, especially in states like California, Florida, and South Carolina
Many of these products still contain active pharmaceutical ingredients (APIs), which persist in the environment and are detectable in waterways downstream from health facilities.
Do not flush any non-hazardous medications unless explicitly listed on the FDA flush list (and even then, local laws may override).
Use designated pharmaceutical waste containers, labeled for non-hazardous meds.
Segregate from hazardous waste, sharps, chemo, and biohazard containers.
Document quantities and types of waste generated, especially if reporting is required by local or state health departments.
When possible, use reverse distribution services to return unopened and unexpired non-hazardous drugs.
Treat all non-hazardous pharmaceuticals with the same rigor as hazardous ones:
Keep locked and labeled containers in secure areas
Train staff on what qualifies as non-hazardous
Use DEA-registered reverse distributors even for OTC medications, to ensure proper tracking and destruction
Retail and independent pharmacies (expired OTC inventory)
Long-term care facilities (medications no longer prescribed)
Veterinary clinics (non-hazardous supplements and meds)
Hospitals and clinics (outpatient prescriptions and OTC stock)
Partner with a provider like Easy Rx Cycle that uses eco-friendly incineration or waste-to-energy conversion, minimizing landfill waste and carbon impact even when handling non-hazardous materials.
Controlled substances are among the most tightly regulated forms of pharmaceutical waste. These drugs have a high potential for misuse, diversion, or abuse and fall under the jurisdiction of the Drug Enforcement Administration (DEA). They are classified into five schedules (I–V) based on their medical use and potential for dependency or abuse.
If your facility handles, stores, dispenses, or disposes of controlled substances, you are considered a DEA registrant and must follow strict federal protocols for proper disposal, including specific documentation, secure storage, and chain-of-custody verification.
The DEA schedules controlled substances into five categories:
Schedule I: No accepted medical use; highest abuse potential (e.g., LSD, heroin, ecstasy – not typically found in healthcare settings)
Schedule II: High abuse potential; tightly regulated (e.g., fentanyl, morphine, hydromorphone)
Schedule III: Moderate to low physical dependence potential (e.g., ketamine, buprenorphine)
Schedule IV: Low abuse risk (e.g., diazepam, lorazepam)
Schedule V: Lower potential for abuse (e.g., pregabalin, certain cough preparations)
Some states treat medications differently than the federal schedule. For example, gabapentin (Neurontin) is a Schedule V controlled substance in some states due to increased concern about misuse in both human and veterinary practices.
Fentanyl – Potent opioid analgesic (Schedule II)
Hydromorphone (Dilaudid) – Schedule II opioid used for severe pain
Morphine – One of the most widely prescribed Schedule II opioids
Ketamine – Dissociative anesthetic (Schedule III), often used in both human and veterinary applications
Diazepam – Anti-anxiety Schedule IV drug
Phenobarbital – Common in both hospital and veterinary clinics (Schedule IV)
Disposal of controlled substances must follow DEA protocols, whether they are expired, damaged, or partially used for disposal. You cannot dispose of these medications through trash, flushing, or regular medical waste channels.
You must use a DEA-registered reverse distributor like Easy Rx Cycle, which provides compliant collection, documentation, and destruction services.
Here’s what’s required:
Required when controlled substances are rendered non-retrievable (destroyed)
Must include:
Drug name and quantity
Method of destruction
Date and location
Names and signatures of two authorized witnesses
Must be submitted to the DEA or retained depending on your facility type and state law
Before destruction or pickup, controlled substances must be:
Stored in a DEA-compliant safe or lockbox
Monitored with limited access
Logged in a controlled substance logbook
Only DEA-registered reverse distributors can take custody of Schedule I–V substances for the purpose of disposal.
Easy Rx Cycle handles:
DEA Form 222 (for CII transfers)
Chain-of-custody documentation
Sealed, tamper-evident packaging
Final incineration at certified destruction facilities
Certificates of Destruction (CODs)
All destruction events must be observed by two authorized individuals:
Typically licensed staff members (pharmacist, nurse, veterinarian)
Both witnesses must sign the DEA Form 41 or similar internal record
Hospitals and surgery centers – High volumes of Schedule II–IV drugs
Pharmacies – Must manage expired and returned controlled substances
Long-term care facilities – Handle PRN or discontinued prescriptions
Veterinary clinics – Must track euthanasia agents and sedatives
Compounding pharmacies (503A and 503B) – Handle Schedule II APIs
Research labs and biotech facilities – Often work with small quantities of regulated drugs
Failing to follow DEA rules can result in:
DEA registration suspension or revocation
Civil fines and criminal penalties
Employee termination or licensing violations
Drug diversion incidents and loss of public trust
Even the improper disposal of a small volume of controlled substances—such as flushing opioids or mixing them with trash—can trigger a full DEA audit.
Whether you’re disposing of Schedule II opioids or Schedule V anti-seizure medications, Easy Rx Cycle ensures every detail meets DEA, EPA, and state law.
Compounded medications are custom-made drugs prepared by licensed pharmacists or compounding pharmacies to meet the specific needs of individual patients. These formulations may involve unique combinations, concentrations, or delivery methods not available in standard commercial drugs. They are prepared in two types of facilities:
503A Compounding Pharmacies: Prepare medications for individual patients based on a valid prescription.
503B Outsourcing Facilities: Manufacture larger batches of compounded drugs for healthcare facilities without needing patient-specific prescriptions. These facilities are subject to FDA oversight under the Drug Quality and Security Act (DQSA).
While compounded medications often improve patient care, they also introduce significant pharmaceutical waste challenges, especially when they involve hazardous drugs, controlled substances, or highly potent ingredients.
Many compounded drugs are made using:
Active Pharmaceutical Ingredients (APIs) in raw form
High-potency agents that require trace-contamination control
Controlled substances regulated by the DEA
Sterile ingredients with short shelf lives or high risk of contamination
These factors increase the likelihood that compounded medications will:
Become pharmaceutical waste before use (e.g., unused syringes, expired vials)
Require hazardous waste disposal due to toxicity or flammability
Fall under both RCRA and DEA regulations, depending on formulation
Partial vials of sterile injectable compounds (e.g., morphine, ketamine)
Expired or unstable batches that fail quality assurance testing
Contaminated ingredients or containers
Waste from cleanroom environments, including gloves, wipes, and gowns contaminated with hazardous APIs
Controlled substances in compounded form, such as compounded oral suspensions or injectables
503A and 503B facilities must develop comprehensive programs for managing and disposing of:
Hazardous waste pharmaceuticals (EPA, RCRA)
Controlled substances (DEA)
Non-hazardous compounded waste (state/local regulations)
Segregation of hazardous vs. non-hazardous waste at the compounding site
Use of secure waste containers—chemically compatible, clearly labeled, and stored per USP <800> standards (for hazardous drugs)
DEA Form 222 and Form 41 compliance for controlled substance components
Use of licensed reverse distributors for transport and destruction
Detailed logs and manifests for every type of waste generated
Document Everything
Track batch numbers, lot numbers, ingredients, quantities, expiration dates, and compounding logs alongside waste manifests.
Isolate Hazardous APIs
Many APIs used in compounding are listed by NIOSH (e.g., cyclophosphamide, methotrexate). Waste containing these must be incinerated.
Minimize Wastage During Production
Adopt lean compounding procedures to reduce the volume of pharmaceutical waste generated per batch.
Use DEA-Registered Reverse Distributors
Particularly for 503B facilities, which often handle Schedule II–V substances in bulk. Destruction events must be witnessed and recorded.
Maintain Cleanroom Compliance
All contaminated materials used during compounding (e.g., PPE, containers, tubing) must be treated as hazardous waste when applicable.
503A pharmacies preparing individualized medications for retail patients
503B outsourcing facilities supplying hospitals, surgery centers, and specialty practices
Hospital pharmacies compounding IV bags, chemotherapy regimens, or parenteral nutrition
Veterinary compounding pharmacies creating pet-specific dosages or formulations
Mismanaging compounded waste can lead to:
Cross-contamination risks in cleanroom environments
DEA violations if Schedule I–V drugs are not documented
Environmental harm from improper disposal of APIs
Compliance failure with FDA inspections or state board audits
Whether you’re discarding expired compounded morphine syringes, expired hormone therapy capsules, or partially used chemotherapy solutions, you must follow best practices for proper disposal.
Chemotherapy waste—also referred to as antineoplastic waste—consists of drugs and materials used in the treatment of cancer that are either hazardous, potentially hazardous, or contaminated. These drugs are designed to kill or inhibit the growth of rapidly dividing cells, and while medically critical, they are also highly toxic to humans, animals, and the environment when disposed of improperly.
Chemotherapy waste often overlaps with hazardous waste pharmaceuticals, meaning facilities must follow RCRA regulations and sometimes NIOSH hazardous drug handling guidelines. Even trace-contaminated items—like gloves, gowns, IV lines, or empty vials—are subject to strict disposal rules.
Chemotherapy waste can be divided into two primary categories:
Includes:
Partially used or expired chemotherapy drugs
Vials or syringes containing more than “trace” amounts of drug
Spill clean-up materials
Mixed solutions with cytotoxic drugs
These often qualify as RCRA hazardous waste pharmaceuticals and must be treated accordingly:
Stored in labeled, leak-proof, hazardous waste containers
Transported by licensed hazardous waste haulers
Destroyed via high-temperature incineration at RCRA-permitted facilities
Includes:
Empty IV bags, tubing, gloves, gowns, and wipes that have come into contact with chemotherapy agents but contain only minimal residual amounts
PPE and packaging used during administration
While not always regulated as RCRA hazardous, trace chemo waste still requires careful waste management due to the risk of occupational exposure and environmental harm. It must be:
Collected in yellow, clearly labeled containers
Managed separately from other pharmaceutical or regulated medical waste
Incinerated, not landfilled
Cyclophosphamide
Ifosfamide
Methotrexate
Doxorubicin
Cisplatin
Fluorouracil
These agents are also listed on the NIOSH Hazardous Drug List, and many meet RCRA criteria for toxicity and mutagenicity.
Segregation at the Point of Generation
Use designated containers for bulk vs. trace chemotherapy waste
Avoid mixing with other hazardous or non-hazardous pharmaceuticals
Clear Labeling
All containers should be marked “Chemotherapy Waste – Incinerate Only”
Include biohazard labels when trace materials are involved
Use of Personal Protective Equipment (PPE)
Staff involved in administration and disposal should wear NIOSH-approved PPE
Contaminated PPE must be disposed of as trace waste
High-Temperature Incineration
Chemotherapy waste must be sent to approved incineration facilities
Incineration ensures the complete destruction of cytotoxic agents
Employee Training
Train staff to identify bulk vs. trace chemotherapy waste
Reinforce OSHA, NIOSH, and RCRA handling protocols
Documentation
Maintain waste manifests for all hazardous shipments
Keep Certificates of Destruction and disposal logs
Hospitals and Oncology Clinics – High-volume chemotherapy use and complex compounding procedures
Ambulatory Surgery Centers (ASCs) – Outpatient cancer treatment centers must manage both trace and bulk chemo waste
Veterinary Oncology Practices – Many pet cancers are treated with human chemotherapy agents, generating similar waste
503B Compounding Pharmacies – Manufacture chemotherapy drugs in batch form, producing high-potency hazardous waste
Improper chemotherapy waste disposal can:
Cause severe exposure risks for staff and waste handlers
Pollute water systems if flushed or landfilled
Lead to EPA, OSHA, or DOT violations, resulting in steep fines
Compromise the safety of oncology care environments
Many chemotherapy drugs are classified as hazardous pharmaceutical waste under EPA rules and require exact waste disposal protocols.
Pharmaceutical waste is generated by nearly every type of healthcare or life sciences organization. These are called “waste generators,” and they each face unique challenges.
Hospitals are among the largest generators of pharmaceutical waste in the United States. They administer thousands of medications daily across emergency departments, surgical units, intensive care units (ICUs), and oncology departments.
Pharmaceutical Waste Challenges:
High volumes of both hazardous pharmaceutical waste (e.g., chemotherapy, opioids) and non-hazardous waste (e.g., OTC meds)
Need for rigorous chain-of-custody logs, especially when managing controlled substances like morphine or fentanyl
Must comply with the Resource Conservation and Recovery Act (RCRA), DEA, and state-specific waste regulations
Must train dozens to hundreds of employees on proper disposal and waste segregation
Hospitals typically work with reverse distributors and specialized waste vendors like Easy Rx Cycle to handle segregation, tracking, and incineration.
Outpatient surgery centers generate pharmaceutical waste primarily through the use of anesthesia, controlled pain medications, and procedural drugs.
Pharmaceutical Waste Challenges:
Frequent handling of Schedule II controlled substances (e.g., ketamine, hydromorphone), requiring secure storage and documented disposal
Limited staff with expanded responsibilities—making streamlined waste management systems essential
Pressure to remain compliant while keeping operational costs low
Because ASCs deal with smaller volumes, they often rely on DEA-compliant mail-back kits or scheduled pickups for waste disposal.
Pharmacies play a critical role in the pharmaceutical waste disposal process, not only by generating waste but also by accepting returns and managing recalls.
Pharmaceutical Waste Challenges:
Expired or damaged medications, sometimes including hazardous waste pharmaceuticals
Handling of controlled substances that must be disposed of using DEA Form 41
Pressure to avoid improper disposal (e.g., flushing medications or throwing them in the trash), which can violate waste regulations
Navigating state regulation differences when operating across multiple jurisdictions
Retail and hospital pharmacies often benefit from pharmacy waste management programs that include:
Reverse distribution services
Medication mail-back solutions
Detailed compliance documentation
Easy Rx Cycle supports pharmacies with custom pickup schedules, secure return packaging, and flat-rate pricing for all categories of waste.
Veterinary clinics often use many of the same pharmaceuticals as human medicine—including opioids, sedatives, and antimicrobials—making them significant generators of both hazardous waste pharmaceuticals and controlled substances.
Pharmaceutical Waste Challenges:
Schedule II–V controlled substances used in sedation, pain relief, and euthanasia
Must maintain a controlled drug log and ensure proper disposal in accordance with state veterinary boards
Frequent use of medications like gabapentin (Neurontin), which is a Schedule V controlled substance in some states
Managing expired vaccines, injectables, and topicals, all of which require different disposal methods
Veterinary clinics often operate with limited staff and storage, so mail-back programs and compact waste containers are essential.
Several federal and state agencies oversee managing pharmaceutical waste:
Proper pharmaceutical waste disposal is governed by a complex framework of federal laws, state regulations, and agency-specific rules. Healthcare facilities, pharmacies, veterinary practices, and research labs must navigate this regulatory environment to remain compliant, avoid fines, and protect public health.
Here are the key regulatory bodies and frameworks you need to understand when managing pharmaceutical waste in the Southeast and beyond.
The EPA is the primary federal agency responsible for regulating hazardous pharmaceutical waste under the Resource Conservation and Recovery Act (RCRA). The EPA’s pharmaceutical rule (40 CFR Part 266 Subpart P) applies to most healthcare facilities and reverse distributors.
Defines hazardous waste pharmaceuticals as drugs that are toxic, corrosive, ignitable, or reactive
Prohibits the sewering or flushing of hazardous pharmaceuticals
Requires proper segregation, labeling, and storage in leak-proof, closed containers
Establishes requirements for manifesting, tracking, and final destruction
Requires generators to properly categorize waste as either acute hazardous waste (P-list) or non-acute (U-list)
Warfarin (P001)
Nicotine (P075)
Lindane (U129)
Chloral hydrate (U034)
If your facility generates these, even in small amounts, you are subject to hazardous waste regulations under RCRA.
The DEA regulates the management and disposal of controlled substances (Schedules I–V), including the movement, storage, and destruction of expired or unused controlled drugs. Improper disposal or incomplete records can trigger audits, fines, and criminal liability.
Form 222 – Required for Schedule II controlled substance transfers (e.g., morphine, hydromorphone)
Form 41 – Required to document the destruction of any Schedule I–V controlled substances
Reverse Distribution – DEA requires that only DEA-registered reverse distributors may receive, handle, and destroy controlled substances on behalf of a registrant
Witnessed Destruction – Destruction must be verified by two authorized staff members, and documented in compliance with state and federal law
Secure Storage – Controlled substances awaiting disposal must be locked in DEA-compliant storage
Failing to follow DEA protocols may result in the loss of licensure and federal prosecution.
While OSHA doesn’t regulate pharmaceutical waste directly, it sets safety standards related to the handling, labeling, and disposal of hazardous drugs. These rules are critical for employee safety in healthcare, pharmacy, and lab settings.
Enforce compliance with Hazard Communication Standard (HCS) for labeling
Mandate use of PPE when handling hazardous drugs, especially antineoplastics and cytotoxic agents
Require documentation and training on spill response, waste handling, and employee exposure prevention
Work in tandem with NIOSH guidelines for managing hazardous drugs in clinical and compounding environments (e.g., USP <800>)
Facilities that do not comply with OSHA standards may face penalties and workplace safety violations.
While federal agencies set the baseline for pharmaceutical waste disposal, state environmental agencies in the Southeast add their own compliance layers. These vary by state and often include reporting, licensing, inspection, and tracking requirements.
Below are examples from key states in the region:
Requires hazardous waste manifesting, even for small-quantity generators
Enforces detailed labeling requirements for containers
State inspectors may request records of hazardous waste pickups and disposal history
Facilities must submit waste disposal reports for review
Mandates inventory tracking of pharmaceutical waste, especially from hospital and pharmacy settings
Regulated facilities must submit periodic waste disposal reports
Waste disposal vendors must be registered with the Georgia Environmental Protection Division
Conducts regular facility inspections for pharmaceutical waste generators
Tracks the generation of hazardous waste pharmaceuticals in outpatient facilities, research labs, and LTC homes
Requires container labeling and documentation audits
Requires generator permits for facilities that exceed monthly thresholds of hazardous waste
Waste transporters must hold state-issued licenses
Facilities must maintain detailed waste logs and submit compliance documentation annually
Together, these agencies ensure that pharmaceutical waste is:
Safely segregated and stored
Properly documented and transported
Destroyed in a manner that prevents environmental harm and drug diversion
Failure to comply with these regulations can result in:
Fines reaching tens of thousands of dollars
DEA license revocation
EPA hazardous waste violations
OSHA citations and employee safety risks
State enforcement actions, permit suspensions, or public reporting penalties
Controlled substances must always be:
Separated from all other pharmaceutical waste
Placed in clearly labeled containers specifically designated for controlled drugs
Never mixed with hazardous waste pharmaceuticals or regulated medical waste
This prevents accidental co-mingling and ensures proper handling at every stage. For example:
Schedule II opioids (e.g., hydromorphone) should never be discarded with chemotherapy waste
Gabapentin (Neurontin), while not federally scheduled, is a Schedule V controlled drug in many states and must be segregated as required by state regulations
Controlled substances awaiting disposal must be stored in locked, tamper-proof storage in accordance with DEA regulations.
Requirements include:
Limited access: Only authorized personnel may handle or access these substances
Physical safeguards: Storage must meet the DEA's safe requirements (21 CFR § 1301.72)
Inventory control: Facilities must reconcile storage logs with dispensing logs to detect any discrepancies
This is particularly critical in veterinary clinics, long-term care facilities, and rural clinics, where storage may be decentralized or shared between departments.
Every controlled substance destined for disposal must be entered into a Controlled Substance Log (also called a Destruction Log or Drug Disposal Record).
Required logging details include:
Date and time of disposal preparation
Name and strength of the medication
Quantity to be disposed
Reason for disposal (expired, recalled, discontinued)
Names of two authorized witnesses
Any DEA Form numbers used in the transfer or destruction
Logs must be stored for at least two years, and many facilities store both digital and hard-copy versions to ensure redundancy during inspections.
Destruction must occur using a DEA-approved method and meet the DEA standard of non-retrievable destruction—meaning the substance cannot be physically or chemically reconstructed or diverted.
Common methods include:
High-temperature incineration
Chemical digestion
Commercial destruction through a registered reverse distributor
Facilities cannot flush controlled substances or discard them into regulated medical waste unless the DEA explicitly permits it under exceptional circumstances (e.g., immediate threat to life).
Before destruction, DEA Form 41 must be completed and submitted (or retained, depending on DEA registrant type and state law). Form 41 must include:
Drug name, strength, and quantity
Method of destruction
Date and location of destruction
Two witness signatures
Easy Rx Cycle manages this process from start to finish, providing compliant destruction methods and facilitating all required documentation.
Once destruction is complete, facilities must receive and retain a Certificate of Destruction (COD) or final documentation from the DEA-registered reverse distributor.
This document includes:
Date of destruction
Destruction method
Location of destruction facility
Reference to DEA Form 222 (if CII drugs were transferred)
Signatures of responsible parties
Documentation must be readily available during audits and must align with previously submitted logs and DEA forms.
Any DEA-registered entity or organization that handles Schedule I–V substances must comply, including:
Hospitals
Retail and mail-order pharmacies
503A and 503B compounding facilities
Veterinary clinics
Long-term care and assisted living centers
Biotech labs and research universities
Improper disposal of controlled substances can result in:
DEA registration suspension or revocation
Civil fines and criminal prosecution
State board investigations
Negative audit findings during regulatory inspections
Even unintentional errors—such as an incomplete DEA Form 41 or missing witness signature—can trigger costly corrective actions and reputation damage.
To stay compliant, reduce risk, and protect the environment, healthcare and life sciences facilities must implement a sustainable and compliant pharmaceutical waste management program. Whether you’re a hospital, pharmacy, long-term care facility, or veterinary clinic, these best practices for pharmaceutical waste disposal will help you meet federal and state regulations while also maintaining operational efficiency.
Implementing these steps not only ensures regulatory compliance with agencies like the EPA, DEA, and state boards—but also builds a culture of responsibility that reduces fines, improves safety, and supports environmental sustainability.
Segregation is the foundation of safe and compliant pharmaceutical waste disposal. Improper segregation increases the risk of cross-contamination, environmental harm, and regulatory violations.
Use color-coded, clearly labeled containers at the point of generation
Keep pharmaceutical waste separate from sharps, regulated medical waste (RMW), and biohazards
Segregate into the following categories:
Hazardous waste pharmaceuticals (e.g., warfarin, epinephrine, nicotine)
Non-hazardous medications (e.g., saline, antibiotics, OTC drugs)
Controlled substances (DEA Schedules I–V)
Chemotherapy or trace chemo waste
Use locked containers for controlled substances and maintain secure access
Proper segregation ensures that each waste stream is managed, transported, and destroyed according to the correct federal and state waste regulations.
Even the best-designed waste management program will fail if staff are not properly trained. Comprehensive training reduces mistakes, improves compliance outcomes, and enhances staff safety—especially when handling hazardous pharmaceutical waste and controlled substances.
DEA compliance training on using Form 41 and Form 222
Recognizing hazardous waste pharmaceuticals under RCRA and EPA rules
Understanding state-specific waste disposal regulations
Identifying improper disposal practices (e.g., flushing, trashing, mixing waste streams)
Communicating the environmental impact of mismanaged drug waste
Spill response and personal protective equipment (PPE) use for hazardous drug handling
Training should be ongoing and refreshed whenever:
New waste types are introduced
Regulatory changes occur
DEA, EPA, or OSHA guidance is updated
Working with a licensed, DEA-registered reverse distributor is essential for compliant and efficient pharmaceutical waste disposal. Reverse distributors specialize in returning or destroying expired, damaged, or unused drugs—particularly Schedule I–V controlled substances—and provide full documentation support.
We manage waste pickup, transport, and destruction
We handle DEA-required forms (Form 222 & Form 41)
We provide Certificates of Destruction and full audit trail documentation
We offer flat-rate pricing, so you know what to expect—no surprise invoices
We serve hospitals, pharmacies, LTCs, veterinary clinics, and biotech labs
In short, Easy Rx Cycle streamlines your entire pharma waste management process while keeping your facility inspection-ready.
📦 Bonus: Ask about our Free Mail-Back Kits for small-quantity waste generators, including veterinary and long-term care facilities.
Thorough record-keeping is critical for staying compliant with RCRA, DEA, and state environmental agencies. Regulatory inspections often begin with a review of your disposal records.
Volume and type of waste generated (e.g., controlled, hazardous, non-hazardous)
Pickup logs, including dates, weights, and transporter info
Certificates of Destruction for all controlled and hazardous substances
Any state-required tracking forms or manifesting documents
Logs should be retained for a minimum of two years, and digital copies are recommended to reduce the risk of loss or damage.
While compliance is the primary concern, sustainability in waste management is increasingly important to healthcare organizations. Choosing green alternatives not only reduces your facility’s environmental footprint—it also enhances public perception and aligns with hospital or corporate ESG goals.
Waste-to-energy incineration – Converts waste into usable energy instead of landfilling
Landfill diversion programs – Reduces environmental load from pharmaceuticals
Green transport options – Fuel-efficient, low-emission vehicles for pickup and delivery
Eco-friendly packaging – Recyclable materials and reduced plastic usage
At Easy Rx Cycle, we offer sustainable disposal methods that comply with EPA and RCRA standards while supporting environmental goals.
When done right, managing and disposing of pharmaceutical waste doesn’t have to be complex or expensive. The five best practices outlined above will help your facility:
Comply with federal and state waste regulations
Avoid costly violations or DEA audits
Protect staff, patients, and the environment
Simplify documentation and tracking
Meet sustainability objectives
By following these disposal practices and partnering with a compliant provider, you’ll ensure your program is efficient, safe, and audit-ready.
Every healthcare facility has different needs when it comes to managing pharmaceutical waste. Waste types vary by service offered, drug schedules handled, volume generated, and state or federal compliance obligations. That’s why a one-size-fits-all approach rarely works.
At Easy Rx Cycle, we customize pharmaceutical waste management solutions for each facility type—whether you’re running a busy hospital, a neighborhood veterinary practice, or a chain pharmacy. Below are examples of how we support compliance and efficiency across different healthcare settings.
Pharmacies face unique challenges in balancing inventory management, drug returns, and compliance with controlled substance regulations. Whether it’s expired stock or DEA-regulated medications, pharmacy waste must be securely handled and well-documented.
Our Pharmacy Waste Solutions Include:
Expired Drug Mail-Back Programs
Easy Rx Cycle offers Free Mail-Back Kits for expired, damaged, or recalled medications. This is ideal for pharmacies that generate small to moderate volumes of waste and want a no-hassle solution with prepaid shipping and full tracking.
DEA Form 222 & 41 Assistance
We help you complete and submit Form 222 for Schedule II transfers and Form 41 for controlled substance destruction. Our system includes double-witness verification and tamper-proof logging.
Inventory Reconciliation Support
We support periodic inventory audits and disposal reconciliation reports to meet state pharmacy board and DEA expectations. This minimizes discrepancies and ensures complete tracking of every controlled substance dispensed or destroyed.
On-Demand or Scheduled Pickup Services
If your pharmacy generates higher volumes of waste, we offer scheduled pickups with flexible container options that meet DEA, DOT, and EPA guidelines.
Our flat-rate pricing and compliance-first service make us the ideal pharmacy waste partner.
Veterinary clinics generate many of the same waste types as human healthcare facilities—including controlled substances, hazardous pharmaceuticals, and non-hazardous medications. However, veterinary teams often operate with fewer staff and less storage space, so simplicity and speed are critical.
Our Veterinary Waste Solutions Include:
Tracking of Schedule V Controlled Substances
Many veterinary drugs fall under Schedule V, including gabapentin (Neurontin). We help your practice track, log, and properly dispose of controlled substances while meeting both DEA and state veterinary board requirements.
Gabapentin and Neurontin Disposal Logging
Even though gabapentin is not scheduled at the federal level, it is classified as a controlled drug in many states, especially in the Southeast. Easy Rx Cycle provides state-compliant disposal tracking, including usage logs, disposal entries, and witness verification.
DEA-Compliant Destruction & Documentation
We provide lockable containers, disposal logs, and Certificates of Destruction to ensure compliance. Our team is trained to work with veterinary-specific protocols and support your staff through on-site pickups or mail-back services.
Mail-Back Kits for Low-Volume Clinics
We offer Free Expired Drug Mail-Back Kits for veterinary offices and mobile vet services that need an affordable and easy solution.
Veterinarians trust us because we understand your workflow—and we make compliance simple.
Larger facilities deal with multiple departments, high patient volume, and diverse waste streams—from hazardous waste pharmaceuticals in oncology to Schedule II anesthetics in surgical suites. They require robust, scalable solutions with centralized reporting and proactive compliance strategies.
Our Solutions for Hospitals & Medical Facilities Include:
Centralized Waste Tracking & Reporting
We help you implement a centralized pharmaceutical waste tracking system that spans all departments. This includes waste type segregation, real-time inventory status, and automated compliance reports for RCRA, DEA, and state audits.
Customized Container Solutions
We provide a range of DOT- and DEA-compliant containers based on your facility’s layout and waste volume. From compact under-counter bins to large-volume bulk totes, our containers are color-coded and leak-resistant, simplifying segregation and minimizing cross-contamination risks.
Flexible Pickup Scheduling
Whether you need weekly, bi-weekly, or monthly pickups, we work around your facility’s schedule to ensure timely removal of waste and full chain-of-custody tracking.
Form Management & Compliance Documentation
Our hospital solutions include:
DEA Form 41 processing for controlled substance destruction
EPA and RCRA manifesting
Container logs and Certificates of Destruction
On-site staff education and mock audit preparation
Specialty Program Support (Oncology, Pharmacy, ICU)
We provide targeted guidance for:
Chemotherapy waste from oncology
Expired narcotics from the OR
Sterile compound waste from hospital pharmacies
Tailoring your pharmaceutical waste management program to your facility type improves:
Regulatory compliance with the DEA, EPA, and local health departments
Operational efficiency through customized containers and scheduling
Cost control, especially for smaller clinics using mail-back kits
Audit readiness, with full logs, destruction certificates, and form management
At Easy Rx Cycle, we don’t believe in cookie-cutter service. Whether you manage 50,000 patients or 50 animals, we design your program around your exact needs.
📥 Request a Quote today to get a waste program tailored to your size, specialty, and compliance requirements.
Improper disposal of pharmaceutical waste isn’t just a risk—it’s a regulatory violation that can lead to massive fines, DEA audits, EPA penalties, and damage to your organization’s reputation. Many facilities, especially smaller clinics and veterinary practices, make avoidable mistakes because of poor training, outdated processes, or lack of support from their waste vendor.
Here are the top pharmaceutical waste disposal mistakes—why they matter, and how to avoid them using compliant, modern waste management practices.
| Common Mistake | Why It’s Dangerous | How to Avoid It |
|---|---|---|
| Flushing Drugs | Violates EPA rules under RCRA; DEA prohibits flushing controlled substances | Partner with a reverse distributor for incineration or use mail-back kits |
| Throwing Meds in Trash | Risk of environmental contamination and drug diversion | Train staff on proper segregation and container use |
| Storing Waste Too Long | Increases diversion risk, potential DEA violations, or expired permits | Set recurring pickup schedules and monitor storage limits |
| Skipping DEA Forms | Violates federal law; can result in DEA fines or license suspension | Always complete DEA Form 41 (destruction) and Form 222 (Schedule II transfer) |
| Using Unlicensed Haulers | Using unregistered transporters violates EPA, DOT, and state laws | Use only licensed reverse distributors like Easy Rx Cycle |
Conduct annual audits of your disposal process
Review controlled substance logs monthly
Assign a compliance officer or point person for waste management
Always check your reverse distributor’s DEA and EPA registration
These precautions significantly reduce the risk of noncompliance, especially for facilities that manage high volumes of hazardous waste pharmaceuticals or controlled substances.
Across the United States—and especially in the Southeast—new laws and regulations will go into effect in 2025 that directly impact how healthcare, biotech, and veterinary facilities manage pharmaceutical waste. These updates are being driven by growing concerns around environmental safety, drug diversion, and inconsistent compliance across facilities.
Understanding these upcoming regulations will help you prepare in advance and avoid penalties.
The Environmental Protection Agency (EPA) is increasing oversight under the Resource Conservation and Recovery Act (RCRA). Facilities that handle hazardous pharmaceutical waste will see stricter enforcement, especially in outpatient and research settings.
More frequent EPA inspections, particularly for outpatient clinics, biotech startups, and small hospitals
Expanded hazardous waste definitions, possibly including more P- and U-listed drugs
Enhanced documentation requirements, including real-time logging of waste generated, stored, and shipped
Mandatory use of closed, leak-proof containers for all hazardous waste pharmaceuticals
The Drug Enforcement Administration (DEA) will strengthen controlled substance disposal protocols across all DEA registrants—including healthcare providers, pharmacies, compounding centers, and veterinary clinics.
Mandatory electronic submission of DEA Form 41 for all controlled substance destruction
Stricter enforcement of chain-of-custody protocols
Tighter rules around storage durations and frequency of destruction
Additional scrutiny for Schedule II–V substance disposal, especially fentanyl and ketamine
All DEA registrants must begin transitioning to electronic form management and audit-ready documentation.
Each state in the Southeast is responding to the federal momentum with their own changes—particularly around tracking, permitting, and transporter registration. Easy Rx Cycle is actively tracking these changes and incorporating them into our services.
Facilities must now pass enhanced permit verification to operate as pharmaceutical waste generators
New inspection protocols focused on documentation, storage conditions, and waste volume thresholds
All waste transporters must follow a new transport logging rule that includes:
Time-stamped pickup data
Waste classification logs
Driver and vehicle tracking
Electronic Waste Tracking Systems (EWTS) are now required for all hazardous waste pharmaceutical generators
Facilities must report all waste disposal transactions to the Georgia EPD in digital format
Audit your current waste program for outdated or noncompliant practices
Upgrade your documentation system to handle electronic DEA forms and state logs
Verify your reverse distributor’s licenses at the federal and state level
Train your team on upcoming regulation changes
Work with a partner like Easy Rx Cycle that is already aligned with 2025’s requirements
Our services are built around full compliance with EPA, DEA, and state regulations—including what's coming in 2025. When you partner with us, we’ll help you:
Transition to electronic DEA forms and tracking
Stay current with state waste reporting requirements
Prepare for EPA and DEA audits
Manage and dispose of all types of pharmaceutical waste—hazardous, non-hazardous, and controlled
📥 Request a Quote today and future-proof your pharmaceutical waste program.
Easy Rx Cycle is the Southeast’s most trusted partner for safe, compliant, and cost-effective pharmaceutical waste disposal. We specialize in customized, full-service solutions tailored to the unique needs of healthcare providers, pharmacies, veterinary practices, and biotech facilities throughout Florida, Georgia, Alabama, Arkansas, South Carolina, and beyond.
Whether you're operating a single-location retail pharmacy or managing a multi-site health system, we ensure your waste program meets every federal and state requirement—while minimizing your workload and eliminating unnecessary costs.
✔ DEA-Registered Mail-Back and Pickup Services
We are licensed to handle Schedule I–V controlled substances and all forms of pharmaceutical waste. Choose between:
Secure mail-back kits for expired drugs (perfect for low-volume clinics)
Scheduled pickup programs for hospitals, LTCs, and pharmacies
✔ Secure, DOT-Approved Containers
Our waste containers meet all Department of Transportation (DOT) standards. Options include:
Lockable bins for controlled substances
Color-coded containers for hazardous and non-hazardous waste
Sharps and trace-chemo containers available upon request
✔ Flat-Rate Pricing With No Hidden Fees
Avoid unpredictable charges. We provide:
Transparent, flat-rate service plans
Customized pricing for multi-site healthcare organizations
No surprise invoices, fuel surcharges, or admin fees
✔ Automated Waste Tracking & Reporting
We offer:
Digital dashboards to monitor waste generated
Certificate of Destruction (COD) management
Auto-filled DEA forms (Form 222 and Form 41)
Ready-to-print audit documentation
✔ 100% Compliance With Federal and State Regulations
Our services meet or exceed all current and upcoming laws, including:
Resource Conservation and Recovery Act (RCRA)
DEA regulations for controlled substance disposal
Environmental Protection Agency (EPA) hazardous waste rules
State-specific waste regulations in the Southeast
Healthcare and veterinary organizations choose us because we make compliance simple, reliable, and affordable:
No more confusion around DEA forms or RCRA manifesting
No more stress about state inspections or audit readiness
No more wasting time chasing after Certificates of Destruction
No more inconsistent pricing or hidden fees
At Easy Rx Cycle, we don’t just pick up your waste—we build a solution that fits your workflow, compliance needs, and budget. Whether you're disposing of hazardous pharmaceutical waste, expired medications, or controlled substances, we’ve got you covered.
📦 Free Mail-Back Kits Available
🔒 DEA & EPA Certified
💼 Trusted by Providers Across the Southeast
📥 Request a Quote today and experience the Easy Rx Cycle difference.
Let’s build a better waste program—the compliant, cost-effective, and stress-free way.
Clear answers to common questions about pharmaceutical waste disposal, DEA compliance, and waste management regulations in the Southeast:
Q: What laws govern hazardous pharmaceutical waste?
A: Hazardous pharmaceutical waste is regulated under the Resource Conservation and Recovery Act (RCRA), specifically Subpart P, which was created by the Environmental Protection Agency (EPA) to improve compliance and environmental safety in healthcare settings. This regulation prohibits sewering (flushing) hazardous pharmaceuticals and sets standards for labeling, storage, and transport.
For controlled substances, regulation falls under the Drug Enforcement Administration (DEA). These drugs must follow strict chain-of-custody procedures and require approved destruction methods documented on DEA Form 41.
Q: Is gabapentin a controlled drug in veterinary use?
A: Federally, gabapentin (Neurontin) is not scheduled by the DEA. However, many U.S. states—including in the Southeast—now classify gabapentin as a Schedule V controlled substance due to its potential for abuse and its use in treating chronic pain in animals and humans.
If you're a veterinary practice in a state that schedules gabapentin, you'll need to:
Track its usage in a controlled drug log
Document its disposal
Use DEA-compliant destruction methods (e.g., through a licensed reverse distributor like Easy Rx Cycle)
Q: Can I flush medications down the drain or toilet?
A: Absolutely not. Flushing medications is considered improper disposal under EPA and state environmental protection laws, and it's also a violation of RCRA Subpart P.
Flushed drugs contaminate waterways, disrupt ecosystems, and increase community health risks. Whether you’re disposing of expired chemotherapy drugs, antibiotics, or unused opioids, the only legal and compliant option is to use:
A DEA-registered reverse distributor
Secure mail-back kits
Or an EPA-approved incineration service
Easy Rx Cycle provides all of these compliant solutions.
Q: What forms do I need for DEA compliance when disposing of controlled substances?
A: You need to complete the following DEA forms:
DEA Form 222: Required for the transfer of Schedule II controlled substances to a licensed reverse distributor or disposal vendor. This form verifies the recipient’s DEA registration and ensures legal chain-of-custody.
DEA Form 41: Required for the destruction of any controlled substances (Schedules I–V). This form must be signed by two authorized witnesses and submitted electronically (as of 2025) to the DEA.
Your disposal partner (like Easy Rx Cycle) should assist you in completing, submitting, and retaining these forms for inspection purposes.
Q: What happens during a DEA or EPA audit?
A: During an audit, DEA agents, state regulators, or EPA inspectors will evaluate whether your facility has followed all laws related to pharmaceutical waste disposal and controlled substance handling.
They will typically ask to review:
Controlled drug logs
Waste segregation protocols
DEA Form 41 and 222 records
Certificates of Destruction
Reverse distributor licenses
Waste manifests (especially for hazardous waste pharmaceuticals)
Storage practices and security measures
Being unprepared can result in fines, license suspension, or mandatory corrective actions. Working with a fully licensed, compliant vendor like Easy Rx Cycle ensures you’re always audit-ready.
Need help preparing for an audit or setting up your compliant waste program?
📥 Request a Quote and let Easy Rx Cycle handle the hard parts—so you can stay focused on care.
📬 Ready to protect your facility and eliminate compliance risks?
✅ Request a Quote from Easy Rx Cycle
📦 Order Free Expired Drug Mail-Back Kits
📘 Download Our Disposal Readiness for Controlled Substances
Let’s build your customized pharmaceutical waste management program—compliant, cost-effective, and scalable.

Safe Disposal of Expired Drugs for Healthcare Facilities Proper drug disposal isn't just a best practice—it’s the law. If you work in a hospital,...
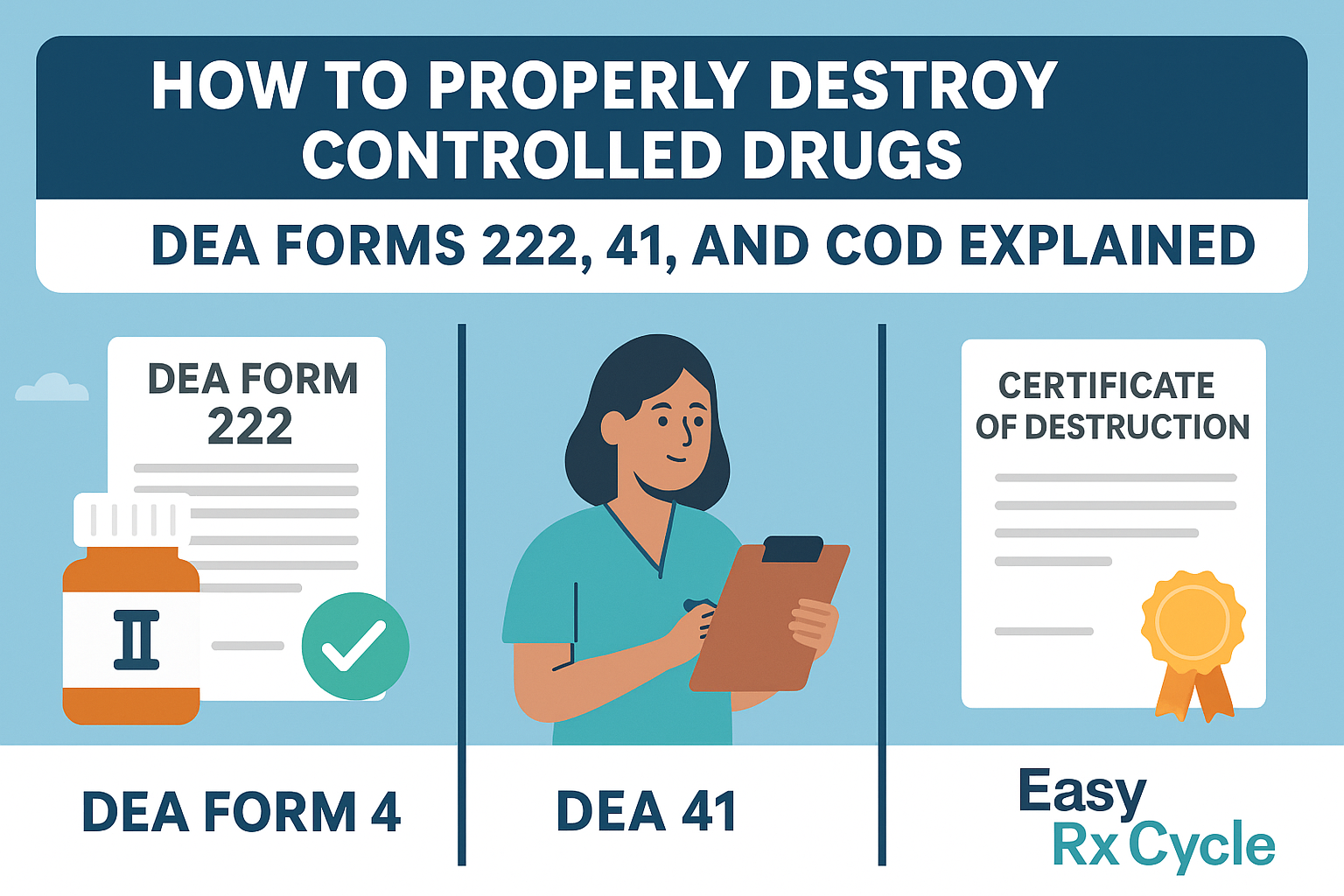
Controlled Drug Destruction: DEA Forms 222, 41, and Certificates of Destruction (COD)
What Is a Reverse Distributor? A reverse distributor is a DEA-registered company that helps healthcare facilities, pharmacies, and DEA registrants...